It’s that time of year again: fall has arrived. The temperature is dropping, the leaves have all turned, and the holiday season is fast approaching. When I think of fall, I remember all of the fun fall activities that I used to do as a kid. My parents would take my brother and I to the pumpkin patch every year and we would choose our pumpkins, argue about whose was better, go on a hayride, and then go home to carve our pumpkins. The other thing that always comes to mind when I think of fall is the food that I associated with fall. I think of hot apple cider, chili, and soup. There is something very cozy and comforting about having a hot meal on a crisp fall day. And one of my family’s favorite fall meals is French Onion Soup, a delicious soup of sweet onions in a rich broth topped with tons of cheese.
French Onion Soup is something that I have eaten many times in my life, but rarely have I stopped to consider the chemistry involved in my dinner. But how is it that the onions become so much sweeter than they ordinarily are? Think about it: raw onions have a sharp taste and a distinct crunch. But onions in French Onion Soup have been caramelized, and as a result they become softer and much sweeter. In the recipe it says that the onions need to be caramelized by cooking them on medium heat for about a half an hour. But what is caramelization? What chemical reaction causes onions to brown and become sweet? And why aren’t cooked onions crisp and crunchy like raw onions? What happens to onions when they are cooked?
One of the chemical processes that occurs while foods are cooking is called pyrolysis. Pyrolysis is a type of non-enzymatic browning in which chemical compounds are broken down by heat without the use of a protein to catalyze or speed up the reaction. When caramelizing onions, pyrolysis is responsible for breaking down sugars using heat, meaning that the high heat applied to the onions during cooking causes the sugars present to break down into smaller units.
All living things break down sugars for energy, including the plants that we eat. In an average medium-sized onion, there are 9 g of sugars. These sugars can be many different from monsaccharides (one single sugar molecule) to disaccharides (two sugars linked together) to polysaccharides (many sugars linked to each other). Plants can make the monosaccharide glucose through photosynthesis. Plant cells can also break down sugars for energy, like all other living things. And polysaccharides are found mainly in the structural components of plants, like the cell walls.
When onions are being cooked, the heat from the pan raises the temperature within the cells of the onion. Once the temperature is high enough, the poly- and disaccharides in the onion are broken down into monosaccharides by breaking the bonds that link the monosaccharides to each other. This reaction of breaking down larger sugars into single sugar molecules is what causes sautéed or caramelized onions to brown and develop a sweeter flavor.
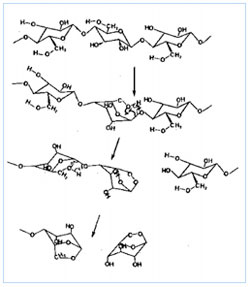
Heat causes the sugar molecules that make up polysaccharides (at top) to separate into monosaccharides (at bottom) in a process called pyrolysis.
Pyrolysis also explains why onions become softer when they are cooked. Much of the structure of plants is made up of starches, which are polysaccharides. When these are heated, they also break down into monosaccharides. So cooking vegetables breaks down the structural sugars present as well, making them softer than raw vegetables.
So the next time that you are sautéing onions for French Onion soup, or browning any vegetable for that matter, think about what is happening to your food at the molecular level. The heat that the stove supplies is breaking down the larger sugars naturally found in vegetables down into monosaccharides, making them taste sweeter and making them softer.
Links:
- French Onion Soup recipe: http://www.foodnetwork.com/recipes/tyler-florence/french-onion-soup-recipe2/index.html
- Sugar Content of an Onion: http://whatscookingamerica.net/onion.htm
- Caramelization Temperatures: http://www.food-info.net/uk/colour/caramel.htm
- Pyrolysis: http://www.cpeo.org/techtree/ttdescript/pyrols.htm
- Pyrolysis (Wikipedia): http://en.wikipedia.org/wiki/Pyrolysis
- Kinetics of Non-enzymatic Browning in Onion Slices
During Isothermal Heating: http://www.sciencedirect.com/science/article/pii/026087749590054F - Formation of Sucrose Pyrolysis Products: http://pubs.acs.org/doi/abs/10.1021/jf60161a013
- Effects of pH on Caramelization and Maillard Reaction Kinetics in Fructose-Lysine Model Systems: http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2621.2001.tb08213.x/abstract
Photos:
- http://img4.myrecipes.com/i/recipes/ck/05/01/french-onion-ck-1011280-l.jpg
- http://tadahfoods.com/wp-content/uploads/2011/01/Caramelizedonionswebsite.jpg
- http://www.diseaseproof.com/rawonion.jpg
- http://chemistry2.csudh.edu/rpendarvis/1feb23.gif
- http://www.eng.iastate.edu/biomapreu/images/2009/thermalbiomass.jpg





Hey Courtney,
With the exception of making my stomach growl, your blog post also made me remember a book that I saw once about all the chemical processes that go in the kitchen called…”Kitchen Chemistry”:
I think this is a perfect blog about college chemists that are soon to be in grad school and cooking for themselves. I find it really interesting to know what is going on with the food that I am making and its funny because as you would agree most chemistry labs are very similar to being in the kitchen (a tad more precise though!). It makes me wonder if the good chefs in restaurants actually know some of the chemistry behind what they are cooking. Also begs the question if its a good idea to improve cooking by finding the science of what is delicious to people and trying to optimize the food so that we are seeking healthy and tasty food.
Brings me to the negative side of this response – fast food. I think that understanding the biology and chemistry behind food has allowed fast food restaurants to tailor their food (full of artificial sugars) to the population in such a way to make them addicted to it:
http://www.thatsfit.com/2010/03/30/fast-food-is-like-heroin-studies-find/
So there is the ethical issue of how do we use what we know about the chemistry of food because it really could be detrimental to our society if it is abused.
Thanks, I will definitely try those recipes!
-Elias
Hi!
I liked the post, it made me want to go back to childhood and pick out pumpkins again and drink hot chocolate on a cold fall day! In other words, I really liked the lead up in the beginning- it really got at the idea of making this a blog for non-science people and getting them interested in a scientific topic. I also really liked that you were able to use a pretty popular topic- food science, which is seen on popular networks like the Food Network’s show Good Eats with Alton Brown (http://www.foodnetwork.com/good-eats/index.html), and used it to teach people a lesson about sugars and pyrolysis. It was a great way to lead to a more complex molecular discussion. I thought you could even explain the differences in sugars a little bit more if you wanted to – ie why monosaccharides would create a sweeter flavor.
Thanks, I really liked it!
Sarah
I really liked your post Courtney.
I love caramelized onions and other delicious fall foods. I had never really thought about how they got so much sweeter by cooking them, but I enjoy them so much more that way. After reading your post I feel so much smarter and want to share my random chemistry of food knowledge with my roommates.
I also like how you made the post readable to non chemistry majors and really kept me drawn in (just like Alton Brown….yep, I watch Food Network just like Sarah).
Lindsey
Hi Courtney,
I love this post, especially the fact that how much it is related to chemistry. I remember a friend of mine who took Chemistry in Cooking for winter term mentioning to me briefly about the connection between chemistry and food, but it never occurred to me how big the connections are. Looks like we really need to understand science well to know make the best out of food.
I came across this website while I was looking for more connection between science and cooking and found it really helpful. http://www.exploratorium.edu/cooking/. It contains recipes, activities, and Webcasts that help understand the science behind food and cooking. For example, in the science of egg section, it talks about he change in egg proteins when you heat them, beat them, or mix them with other ingredients. I found it a great interaction study, since it also provides recipe you can use.
I also found this link that has a video of a biochemist and cook explains the importance of chemistry in cooking and how chemistry facts can explain why recipes go wrong.
http://www.sciencedaily.com/videos/2009/0112-chemistry_of_cooking.htm
I found it a great way to convince people about the connection between science and cooking. This also makes me think of one of the non-traditional job for science major is chief and it just all makes sense now.
It is a great post and I enjoy it a lot. Thanks Courtney!
-Sunny
This reminds me of a family tradition at my house: making caramels. The recipe has been handed down for generations and certainly requires a lot of chemistry. Caramel candies are made by boiling a mixture of cream, sugar, vanilla, and butter. You have to “caramelize” the sugar separately before adding the other ingredients. So the same browning that occurs from breaking down sugars in onions is exactly what gives the brown color to caramels (the only difference is you are adding the sugar; it isn’t coming from a vegetable). The important thing about caramelization is temperature. In candy making, for instance, you must take a lot of care in making sure the sugar doesn’t “burn,” which basically means the sugars begin to re-crystallize and turn into giant lumps that burn on the pan. Since we are expert caramel makers in my family, we sort of have a feel for how long to allow the sugar to caramelize before adding the butter and vanilla. I have spent many an hour staring at a meat thermometer which we used to monitor the temperature of the mixture. It is interesting to see how this process can be applied to other things like vegetables, and turn out the same sweetness and brown hue.
http://books.google.com/books?id=3fkAazdFhL8C&pg=PA265&dq=caramels+recipe+smoke&hl=en&ei=1SqJTdjBI4K2sAPNheSRDA&sa=X&oi=book_result&ct=result&resnum=3&ved=0CEoQ6AEwAg#v=onepage&q&f=false
Pingback: Bite Sized Wisdom: A caramelized life | Say No To Food Waste